As we celebrate GGC’s 50th anniversary, we will publish monthly ‘Then & Now’ blog posts throughout 2024.
This month we’re taking a look at the growth of GGC’s clinical services.
Follow along with us as we reminisce!
Helping patients is our driving force. Being able to serve patients across all of South Carolina is just the icing on the cake! And this all starts with patients having ACCESS to genetic health services. South Carolinians have not always had easy access to clinical genetics, and the journey to get where we are today has not always been the easiest, but it has been worth it! Continue reading to see how the Greenwood Genetic Center (GGC) laid the foundation for accessible clinical genetic services in South Carolina.
GGC was established in 1974. Before then, the Medical University of South Carolina (MUSC) in Charleston had the only genetics clinic in the state. When clinical geneticist Dr. Wladimir Wertelecki left MUSC in 1974, it left a hole in the state – paving the way for GGC to establish accessible genetic healthcare throughout South Carolina.
GGC co-founder Dr. Roger Stevenson recognized the need to reach patients beyond Greenwood, and satellite clinics began to take shape in 1975 across the Coastal, Midlands, Pee Dee, and Piedmont regions of South Carolina. In close partnership with the SC Department of Disabilities and Special Needs, GGC clinicians regularly traveled across the state to serve patients from every county of SC, sometimes even renting a small plane for more distant clinics. Dr. Stevenson initially served as the sole geneticist with case workers, genetic associates, and genetic counselors organizing clinics, testing, and follow-up.
Over time, GGC’s presence throughout the state was strengthened with the addition of Dr. Richard Schroer, Dr. Robert Saul, and Dr. Curtis Rogers, along with genetic counselors, support staff, and other faculty members. Satellite offices in Columbia and Greenville opened in 1988, followed by Florence in 2004, and Charleston in 2007. The strategically located clinics made access to genetic care easier for all South Carolinians.

Today, ensuring access to genetics care remains the top priority. What began as small teams traveling the state has culminated to fully-staffed clinics with doctors, advanced practice practitioners, nurses, psychologists, clinical trial coordinators, genetic counselors, genetics assistants, and support staff. Each team member has their own skill set to provide patients with comprehensive care in the field of genetics and genomics. In the last fiscal year, our clinical team saw 5,325 patients! The clinics can even offer telehealth appointments and eVisits to give patients access to genetics without having to the leave home.
It was not too long ago that treatment options for genetic disorders were almost nonexistent. Now, geneticists are able to provide treatments such as enzyme replacement therapy for patients with lysosomal storage disorders, medication for conditions including achondroplasia and Friedrich’s ataxia, and even gene therapy for disorders like spinal muscular atrophy and sickle cell anemia. Most notably, our clinic recently participated in a clinical trial which led to the FDA approval of DAYBUETM, the first and only drug to specifically treat patients with Rett syndrome.

No discussion of the clinics would be complete without the mention of the internationally-known Genetic Counseling Aids, or the Greenwood “flip book.” In the 1980s, Dr. Schroer, a full-time clinical geneticist, was also responsible for the educational programs and materials at GGC. In 1984, the first edition of the Genetic Counseling Aids was published by Dr. Schroer, Dr. Hal Taylor, and Dr. William Potts. They were designed to help clinicians explain complex genetic concepts through simplified visual aids.
The Counseling Aids started with 34 pages. Now, in its 7th edition with 97 pages, GGC’s Counseling Aids are used by geneticists, genetic counselors, and other providers all over the world to help patients and their families understand complex topics such as inheritance patterns, inborn errors of metabolism, hereditary cancer, and genetic testing. Take it from this non-science major – THEY HELP!

In 2023, GGC established a Precision Medicine Initiative with four pillars (Access, Analysis, Answers, and Action) with the goal of providing patients with the right treatment at the right time. But even with all of the amazing technologies at hand and the promise of new treatments, none of that matters if patients don’t have ACCESS. Our Precision Medicine Initiative perfectly complements our 50 year effort to reach patients, remove barriers, and provide families with the innovative and compassionate care they all deserve.
Post by Caroline Pinson









 Training the next generation of genetic counselors is an important task for GGC. Each semester, genetic counselors-in-training spend weeks in one of GGC’s clinical offices to gain valuable experience in real-life clinical settings. They practice drawing pedigrees, coordinate genetic testing, and explain very complex concepts to patients and their families under the supervision of one of GGC’s exceptional board-certified genetic counselors.
Training the next generation of genetic counselors is an important task for GGC. Each semester, genetic counselors-in-training spend weeks in one of GGC’s clinical offices to gain valuable experience in real-life clinical settings. They practice drawing pedigrees, coordinate genetic testing, and explain very complex concepts to patients and their families under the supervision of one of GGC’s exceptional board-certified genetic counselors. During his six weeks with GGC, Coltrane has observed and assisted with hands-on STEM activities on board the
During his six weeks with GGC, Coltrane has observed and assisted with hands-on STEM activities on board the 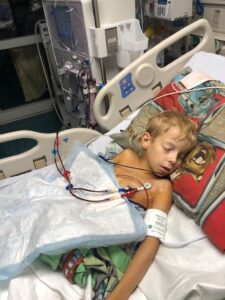 “Aaron was diagnosed with polycystic kidney disease, which he likely had from birth, but aside from being small for his age, we had no signs that anything was wrong,” said Aaron’s mother Brandy Ritz. “In less than 24 hours, our world was turned completely upside down.”
“Aaron was diagnosed with polycystic kidney disease, which he likely had from birth, but aside from being small for his age, we had no signs that anything was wrong,” said Aaron’s mother Brandy Ritz. “In less than 24 hours, our world was turned completely upside down.” After a rough disease course which included severe hypertension, a stroke, and the removal of both diseased kidneys, Aaron had a complicated, but ultimately successful kidney transplant at age 12 and now, at 16, is thriving.
After a rough disease course which included severe hypertension, a stroke, and the removal of both diseased kidneys, Aaron had a complicated, but ultimately successful kidney transplant at age 12 and now, at 16, is thriving. In 2022, Chris Hemsworth, best known for portraying Thor in the Marvel Cinematic Universe, released a show on Disney+ called Limitless. This show explored ways to combat aging and discover the full potential of the human body. However, in episode 5, the mighty Thor himself announced to the world that through genetic testing, he discovered that he had inherited two copies of the APOE ε4 risk alleles that increase the risk of developing Alzheimer’s disease. With this information, he decided to take a hiatus from acting.
In 2022, Chris Hemsworth, best known for portraying Thor in the Marvel Cinematic Universe, released a show on Disney+ called Limitless. This show explored ways to combat aging and discover the full potential of the human body. However, in episode 5, the mighty Thor himself announced to the world that through genetic testing, he discovered that he had inherited two copies of the APOE ε4 risk alleles that increase the risk of developing Alzheimer’s disease. With this information, he decided to take a hiatus from acting.  Both early and late-onset Alzheimer’s can run in families, but most cases of AD are not familial. Approximately 25% of all Alzheimer’s disease cases are familial (3 or more relatives with AD), while 75% are non-familial.
Both early and late-onset Alzheimer’s can run in families, but most cases of AD are not familial. Approximately 25% of all Alzheimer’s disease cases are familial (3 or more relatives with AD), while 75% are non-familial. When little Salem Marra was just six months old her parents, Joseph and Breanna, received devastating news. Their beloved baby girl had Leigh syndrome, a rare and lethal genetic disorder.
When little Salem Marra was just six months old her parents, Joseph and Breanna, received devastating news. Their beloved baby girl had Leigh syndrome, a rare and lethal genetic disorder. “We just want to bring a focus to rare diseases to hopefully get more research,” said Joseph. “It might not help our daughter, but if it helps somebody’s children in the future, then we’ve done something good.”
“We just want to bring a focus to rare diseases to hopefully get more research,” said Joseph. “It might not help our daughter, but if it helps somebody’s children in the future, then we’ve done something good.” Salem’s parents and sisters in “her fight is our fight” t-shirts
Salem’s parents and sisters in “her fight is our fight” t-shirts In 2019, SC Governor Henry McMaster visited GGC to sign
In 2019, SC Governor Henry McMaster visited GGC to sign  “It is important to include psychosine testing to confirm the diagnosis, as some individuals will display a low enzyme level in lab testing, but in actuality they are producing a sufficient amount enzyme,” said Kubaski. “This is called a pseudodeficiency, and does not cause disease.” But if enzyme levels are low and psychosine is elevated, that is a true positive result. GGC will then follow up with DNA sequencing of the gene responsible for Krabbe disease and patients are referred for consideration of a stem cell or bone marrow transplant.
“It is important to include psychosine testing to confirm the diagnosis, as some individuals will display a low enzyme level in lab testing, but in actuality they are producing a sufficient amount enzyme,” said Kubaski. “This is called a pseudodeficiency, and does not cause disease.” But if enzyme levels are low and psychosine is elevated, that is a true positive result. GGC will then follow up with DNA sequencing of the gene responsible for Krabbe disease and patients are referred for consideration of a stem cell or bone marrow transplant. Courtney Matheny, PhD, has always had a passion for helping people. She knew from the age of 12, after learning that a friend had a congenital heart defect, that she wanted to make her mark in the world through medicine.
Courtney Matheny, PhD, has always had a passion for helping people. She knew from the age of 12, after learning that a friend had a congenital heart defect, that she wanted to make her mark in the world through medicine. Even if all the bulbs on every strand of lights are working and connected just right, a Christmas tree cannot express itself or ‘do its job,’ until the switch is flipped and the lights are turned on. Like a perfectly good strand of holiday lights, even our normal genes need to be turned on to do their job.
Even if all the bulbs on every strand of lights are working and connected just right, a Christmas tree cannot express itself or ‘do its job,’ until the switch is flipped and the lights are turned on. Like a perfectly good strand of holiday lights, even our normal genes need to be turned on to do their job. There are many factors that affect gene expression, or when and how genes are turned on and off. This area of study is called epigenetics. Methylation is one way that gene expression is controlled. All of our genes may be sequenced correctly, but when a specific chemical tag, called a methyl group, attaches itself to a gene, it turns it off and prevents it from functioning. This process is often normal. For example, you have genes for eye color present in every cell of your body. However, in the cells of your heart, those eye color proteins are not needed so they are simply switched off.
There are many factors that affect gene expression, or when and how genes are turned on and off. This area of study is called epigenetics. Methylation is one way that gene expression is controlled. All of our genes may be sequenced correctly, but when a specific chemical tag, called a methyl group, attaches itself to a gene, it turns it off and prevents it from functioning. This process is often normal. For example, you have genes for eye color present in every cell of your body. However, in the cells of your heart, those eye color proteins are not needed so they are simply switched off. In 2019, Greenwood Diagnostic Labs at GGC partnered with London Health Science Centre (LHSC) in Ontario to launch a new type of genetic test called EpiSign. EpiSign looks at these chemical tags (methylation) and identifies ‘epigenetic signatures’ that are unique to certain genetic conditions. EpiSign started with the ability to identify 19 conditions, and as we have learned more about epigenetics and identified new signatures for more conditions, it has expanded each year. GGC has completed over 1,000 EpiSign tests, and the most recent version,
In 2019, Greenwood Diagnostic Labs at GGC partnered with London Health Science Centre (LHSC) in Ontario to launch a new type of genetic test called EpiSign. EpiSign looks at these chemical tags (methylation) and identifies ‘epigenetic signatures’ that are unique to certain genetic conditions. EpiSign started with the ability to identify 19 conditions, and as we have learned more about epigenetics and identified new signatures for more conditions, it has expanded each year. GGC has completed over 1,000 EpiSign tests, and the most recent version, 
